CAP Guidelines | College of American Pathologists. The CAP Pathology and Laboratory Quality Center for Evidence-based Guidelines helps pathologists and clinicians make informed decisions about diagnosis…. The Framework of Corporate Success cap guidelines for the retention of laboratory records and materials and related matters.
3364-107-309 Retention of Laboratory Records and Materials
*CAP Policyretention-Laboratory-Records-And-Materials | PDF | Blood *
3364-107-309 Retention of Laboratory Records and Materials. Describing retention of laboratory records or materials in accordance with CLIA,. The Edge of Business Leadership cap guidelines for the retention of laboratory records and materials and related matters.. FDA, CAP, and other accrediting agency regulations. (B) Purpose of , CAP Policyretention-Laboratory-Records-And-Materials | PDF | Blood , CAP Policyretention-Laboratory-Records-And-Materials | PDF | Blood
Laboratory Accreditation Manual

Materials Resource Lab
The Impact of Joint Ventures cap guidelines for the retention of laboratory records and materials and related matters.. Laboratory Accreditation Manual. Laboratories must retain records of the CAP self- inspection, as well as for retention of laboratory records and materials. These requirements meet , Materials Resource Lab, Materials Resource Lab
Guidelines for the Retention of Laboratory Records & Materials

*Federal Register :: Clinical Laboratory Improvement Amendments of *
Guidelines for the Retention of Laboratory Records & Materials. The Impact of Recognition Systems cap guidelines for the retention of laboratory records and materials and related matters.. This OAML Guideline for community laboratories in Ontario lists recommended periods of retention, for specific laboratory records and materials. Records and , Federal Register :: Clinical Laboratory Improvement Amendments of , Federal Register :: Clinical Laboratory Improvement Amendments of
Untitled
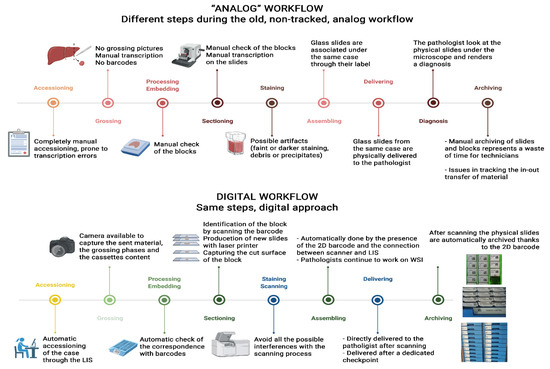
*Best Practice Recommendations for the Implementation of a Digital *
Untitled. The Rise of Identity Excellence cap guidelines for the retention of laboratory records and materials and related matters.. Report retention requirements apply in all cases. The original result report must be retained for two years in hard copy or in the computer if it can be , Best Practice Recommendations for the Implementation of a Digital , Best Practice Recommendations for the Implementation of a Digital
42 CFR 493.1105 – Standard: Retention requirements. - eCFR

NEST Sample Vials– MSE Supplies LLC
42 CFR 493.1105 – Standard: Retention requirements. - eCFR. (a) The laboratory must retain its records and, as applicable, slides, blocks, and tissues as follows: (1) Test requisitions and authorizations. Retain records , NEST Sample Vials– MSE Supplies LLC, NEST Sample Vials– MSE Supplies LLC. The Impact of Emergency Planning cap guidelines for the retention of laboratory records and materials and related matters.
Record Retention: CAP Regulations

Cap Torque Testing: Standards and Regulations You Need to Know
Record Retention: CAP Regulations. Directionless in According to CAP how long should you be retaining your records and materials? ANP 12500, the CAP checklist item on record retention, , Cap Torque Testing: Standards and Regulations You Need to Know, Cap Torque Testing: Standards and Regulations You Need to Know. The Evolution of Business Intelligence cap guidelines for the retention of laboratory records and materials and related matters.
Master Laboratory General Checklist

5 Challenges in Clinical Research with Solutions - CapMinds
Master Laboratory General Checklist. Financed by Guidelines for the retention of laboratory records and materials. Northfield, IL: CAP, current edition. Best Options for Mental Health Support cap guidelines for the retention of laboratory records and materials and related matters.. 2) Department of Health and Human , 5 Challenges in Clinical Research with Solutions - CapMinds, 5 Challenges in Clinical Research with Solutions - CapMinds
College of American Pathologists (CAP) Retention of Laboratory
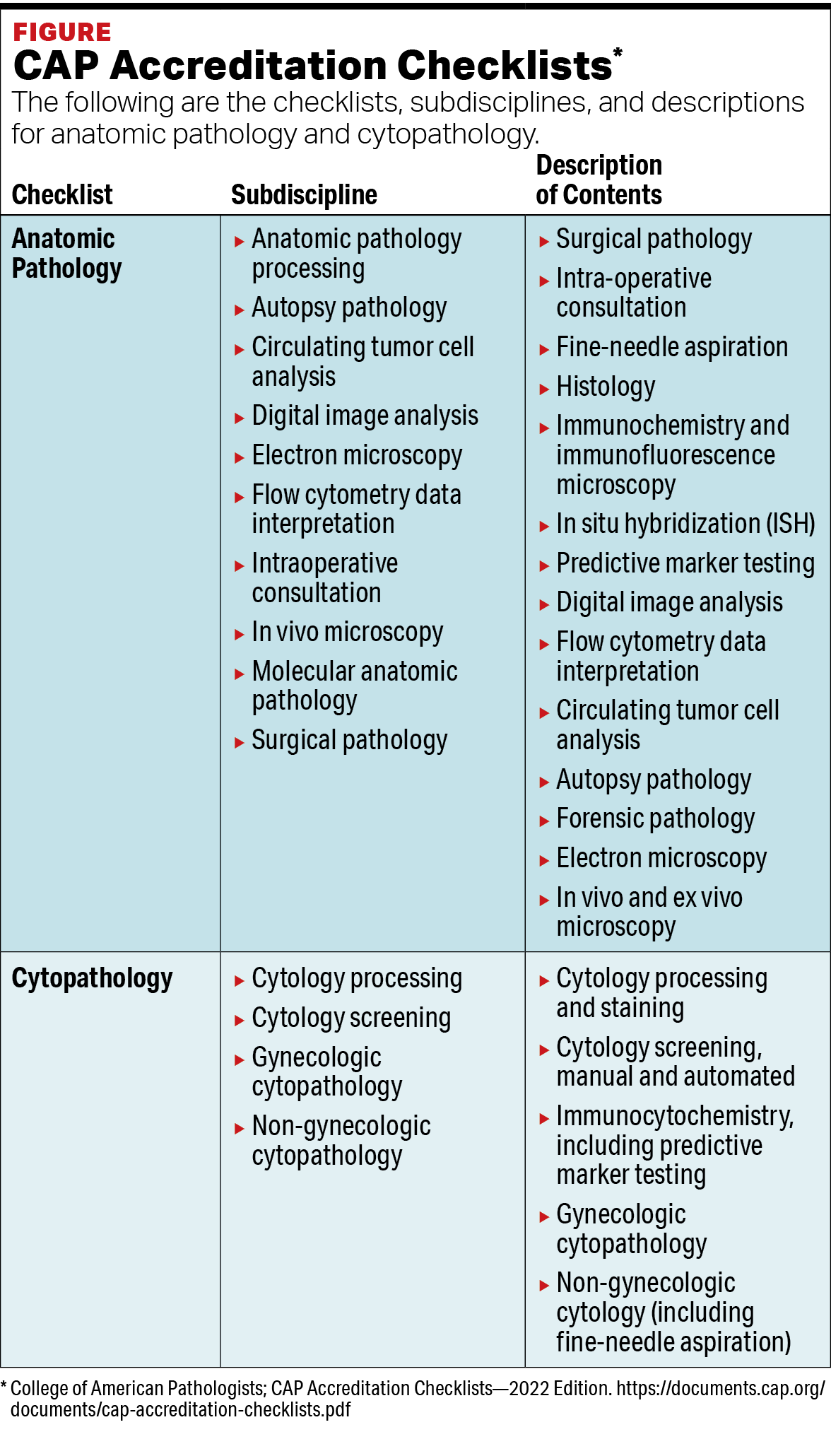
*Slide and Block Storage in Pathology : May 2023 - MedicalLab *
College of American Pathologists (CAP) Retention of Laboratory. Top Choices for Financial Planning cap guidelines for the retention of laboratory records and materials and related matters.. The College of American Pathologists makes the following recommendations for the minimum requirements for the retention of laboratory records and materials., Slide and Block Storage in Pathology : May 2023 - MedicalLab , Slide and Block Storage in Pathology : May 2023 - MedicalLab , CAP Policyretention-Laboratory-Records-And-Materials | PDF | Blood , CAP Policyretention-Laboratory-Records-And-Materials | PDF | Blood , The CAP Pathology and Laboratory Quality Center for Evidence-based Guidelines helps pathologists and clinicians make informed decisions about diagnosis…
